I'm sure you get the idea. There are 5 basic types that most common reactions can be divided into, synthesis, decomposition, single replacement, double replacement and combustion. These sound a lot more confusing than they actually are. We have reactions where things combine, where things come apart, where things change places (2 types) and where things burn. There are also some fancy, complicated organic reactions, but we aren't going to get in to that.
*Reactions where things combine - Synthesis reactions

When two substances (reactants) combine to form a single new compound (product) that is a synthesis reaction. "Synthesis" just means putting things together, and that is all that is happening in these reaction. These are easy to find because they will only have 1 thing after arrow (1 product).
*Reactions where things come apart - Decomposition Reactions
When a single compound (reactant) comes apart and produces 2 or more simpler substances (products) that is a decomposition reaction. Most of us know that decomposition is when stuff comes apart, like whe
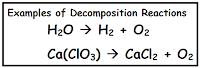 n roadkill on the side of the road slowly gets smaller and smaller over time... You can find these really easily too, just look for reactions with only one thing before the arrow (reactant).
n roadkill on the side of the road slowly gets smaller and smaller over time... You can find these really easily too, just look for reactions with only one thing before the arrow (reactant).*Reactions where things change places - Single and Double Replacement reactions
When you start out with 2 compounds or with 1 compound and 1 element, something is going to change places with something else. These are called replacement reactions because what basically happens is the stronger of the 2 (more reactive) goes in and replaces the weaker (less reactive). It's important to remember that thing only replaces similar things, so metals switch with other metals, nonmetals with nonmetals. We have 2 names for these because 1 describes the 2-compound reactions and one describes the 1-element-and-1-compound reactions.




No comments:
Post a Comment