- All of my students have an email address that I can find
- I can easily set up a website, permanent or temporary, to put out information for specific groups of students
- I can easily set up websites for my students without worrying about them putting information on the internet uncontrolled
- My students can access their assignments in Google Docs from anywhere with internet
- Students can work on the same document or presentation, at the same time, without having to get together in person
- I can have students turn in most of their homework electronically just by emailing it to me and sharing Google Docs with me
- I can easily add comments into assignments submitted through Google Docs and sent those comments back to the students without using any paper
- I can assign any project I want as long as it can be completed using Google Docs, without worrying about which programs my students have on their computers at home
- I can use Forms for in class activities or tests with real-time feedback
Friday, March 26, 2010
Why I love Google Apps for Education
Thursday, March 25, 2010
10 Gaps in Education (that don’t get enough press)
{NOT my own writing, sourced from http://ecologyofeducation.net/wsite/?p=1987 Ecology of Education}
Not all gaps are created equal.
The Achievement Gap gets the most press and seems to have the most leverage. But there are a host of others. Read on for a handy dandy guide to 10 Gaps in Education, and then add your own in the comment section.
Legislative Gap
Distance between lawmakers and teachers.
Potential Gap
Difference between a students’ potential and the tasks they are asked to do.
Stimulant Gap
Time between the bottom of one cup of coffee and a refill. Often described as “The Unbearable Gap”.
The Rumsfeld Gap
The schools, teachers, parents, & students we have vs. the schools, teachers, parents, & students we want.
The Billable Gap
When educators daydream about the money they’d make if they could bill their hours. ”65 hrs this week, times 4 weeks this month, times $125 per hour = Cha-Ching!”
The Arm Chair Gap
When folks with no teaching experience suddenly become experts on issues in education after reading an editorial or article.
The Restraint Gap
What a teacher wants to do vs. What a teacher must do when confronted by someone suffering from The Arm Chair Gap.
The Manchurian Gap
Students who are brainwashed to believe that answering test prep questions actually prepares them for entering the work force.
The Coup d’Gap
When policy makers seize the reins of the education debate by scapegoating and alienating teachers. Often characterized by language centered on market terms — accountability, input/output, achievement — and rigor with little or no mention of students, vigor, or relevance.
The Back-to-the-Future Delorean Gap
Often experienced in schools where there is a mix between 19th century teachers/administrators and 21st century technology proponents.
Wednesday, March 24, 2010
What is your science bias?
Tuesday, March 23, 2010
Women in Science
Monday, March 22, 2010
Problem Solving
Sunday, March 21, 2010
Speaking Up For Scientists
Saturday, March 20, 2010
Where does your waste go?
Friday, March 19, 2010
"Invisibility Cloak" created, no kidding...
Thursday, March 18, 2010
What Males Will Do
Wednesday, March 17, 2010
Science and Writing
Tuesday, March 16, 2010
It's a solid, it's a liquid...it's a non-newtonian fluid!
Everything on the video is real, they really are walking, and falling into, a giant tub of this stuff. It is not movie magic. Maybe it is real magic??? Nah, just awesome science.
Monday, March 15, 2010
Cool Video Competition
The Chemical Heritage Foundation will be launching a national video competition in the fall of 2010. It will be part of the International Year of Chemistry 2011. They will be creating an interactive periodic table, where each element has a short, documentary style video. Students all over the country (and the world?) will be creating these videos and submitting them to the competition. The winners will get to go to Heritage Day 2011 where they will meet the judges and attend an award ceremony.
For more information:
http://www.chemheritage.org/classroom/its-elemental/index.html
Sunday, March 14, 2010
Pi for everyone!
Yes, there is a day dedicated to celebrating the wonderful number pi. You all remember pi from math class? And maybe from rotational motion if you took physics? It has been known for a long time and we just keep getting to know it better. It never repeats and as far as we know it goes on forever. It has been calculated to over a trillion decimal places. That is more than you could even hope to count. It is used by scientists, mathematicians, teachers and even the ever reluctant students to calculate more things than you could even imagine. There is at least one thing you use in your daily life that owes it's existence, or at least it's current better-than-it-used-to-be condition to pi.
So I leave you with pi...to 50 decimal places
3.14159265358979323846264338327950288419716939937510
http://www.piday.org/
Saturday, March 13, 2010
Since when do names include numbers?
Some elements can only have one charge, so you can just use their name without needed to add anything to explain what the charge is. Sodium is always 1+ charge, so you can just name the compounds sodium chloride, sodium oxide, etc.
But some elements can have different charges at different times. Copper is sometimes 1+ and sometimes 2+, so you can't just call something copper oxide. You wouldn't know if that was CuO or Cu2O becuase you wouldn't know what charge to criss-cross. So we use a Roman numeral in the name to show what charge the metal has in this particular substance.
So CuO would be copper II oxide and Cu2O would be copper I oxide. Remember that the Roman numeral doesn't tell us what the subscripts are, they tell us the charge. It's easy to get confused and get the names backwards.
So which elements need these Roman numerals? All of the transition metals except for zinc, cadmium and silver can have more than one charge and need Roman numerals. Those three always have the same charge (silver is 1+ and zinc and cadmium are 2+) so they don't need Roman numerals. Also tin and lead, even though they aren't transition metals, can have 2+ or 4+ charges and need Roman numerals.
Friday, March 12, 2010
Dance Robot Dance!
Super Simple Dancer
Synchronized robots
Japanese Dancing
Thursday, March 11, 2010
15 year old ABE lost at sea
News Release : Pioneering Deep-Sea Robot Lost at Sea : Woods Hole Oceanographic Institution
Wednesday, March 10, 2010
Ionic, covalent, polyatomic...are those even real words?
And then they want you to name the compounds. And if you try to give them nice names like Buttercup or Hugo they don't give you any credit at all... They want you to follow a very confusing set of rules just to give the simple looking symbols long confusing names. Nitrogen triiodide (which by the way is so explosive it will ignite it touched by a feather) has a name that looks like it can't possibly be real, or at least must be spelled wrong. Who puts two "i"s in a row in a word anyways.
The rules don't really have to be that complicated if you break it down in to pieces. First you have to figure out if the compound you have is ionic or covalent (there are also acids, organic compounds and other special compounds, but that is for a different post).
Remember that an ionic compound is made of one positive and one negative ion. That almost always means one metal and one nonmetal. The only common exceptions are hydrogen, which can act like either a nonmetal or a metal depending on the situation, and the polyatomic ions which are a dead giveaway that you have an ionic compound. Hydrogen you can name like a metal in simple compounds.The polyatomic ions each have a special name, like sulfate or hydroxide, and they keep that name in the compound.
Ionic compounds are named by giving the name for metal followed by the name of the nonmetal.
- Ending of the nonmetal is changed to -ide (oxygen becomes oxide, chlorine becomes chloride)
- Polyatomic ions keep their -ite and -ate endings
- If the metal can have more than one charge[transition metals plus tin and lead, minus silver, cadmium and zinc], include the Roman Numeral for the charge it has in that compound
Covalent compounds are made of only nonmetals. Since they can go together in more than one combination (N2O, NO2, N2O3) you can't just use the element names, no one would know which nitrogen oxide you had. So we use prefixes, or extra letters in front of the name, to show how many of that element are in the particular element you are trying to name right now.
So N2O would be dinitrogen monoxide, di- meaning 2 for the two nitrogens, and mono- meaning 1 for the one oxygen. NO2 would be nitrogen dioxide, notice that it isn't mononitrogen. One of the goofy things with the prefixes is that is if the first element is only one, they don't include the mono-, but if the second element is one they do.
Tuesday, March 9, 2010
All models of the atom you have ever seen are wrong...
 So if you know anything about how atoms are actually put together, you know that every model you have ever seen of an atom is wrong. The electrons are too big, too close to the nucleus and usually too planetary-orbit-like. This clip from Nova Science does an excellent job of showing (and fixing) these problems, as well as a few I hadn't thought of before.
So if you know anything about how atoms are actually put together, you know that every model you have ever seen of an atom is wrong. The electrons are too big, too close to the nucleus and usually too planetary-orbit-like. This clip from Nova Science does an excellent job of showing (and fixing) these problems, as well as a few I hadn't thought of before.http://www.youtube.com/watch?v=5fPOCcj7gEU
In reality the real reason the models are wrong is that there isn't any way to make an accurate model that is of a size and scale that people could actually see and use. That's part of the problem with atoms after all, they are very small and very hard to wrap our brains around.
Monday, March 8, 2010
Welcome Copernicium!
 Once upon a time there were some lonely lead atoms in Germany. Then, in February of 1996 some scientists saw that the lead atoms were lonely and decided to introduce them to some friendly zinc atoms. So they loaded up the particle accelerator and played nuclear matchmaker. Much to the scientists delight, the lonely lead atoms and the friendly zinc atoms got along really well. So well in fact that their nuclei combined together to make the very first atoms of brand spanking new baby element, number 112. Baby 112 didn't last very long, only fractions of a second, but it's birth meant the world had yet another new element to name.
Once upon a time there were some lonely lead atoms in Germany. Then, in February of 1996 some scientists saw that the lead atoms were lonely and decided to introduce them to some friendly zinc atoms. So they loaded up the particle accelerator and played nuclear matchmaker. Much to the scientists delight, the lonely lead atoms and the friendly zinc atoms got along really well. So well in fact that their nuclei combined together to make the very first atoms of brand spanking new baby element, number 112. Baby 112 didn't last very long, only fractions of a second, but it's birth meant the world had yet another new element to name.
And nearly 14 years later little element 112 was still unnamed. Relegated to the temporary name of "ununbium" and confusing symbol of Uub. And now, finally, little 112 has a name! I would like to introduce you to Copernicium!! Named after Nicolaus Copernicus, on February 19, 2010 the IUPAC finally agreed on the name and symbol (Cn because Cp was too easily confused with other scienc-ey symbols).
So let us all welcome yet another element about which high school students will ask "what it is?" and we will say "it the element fill-in-the-blank" and they will say "no, I mean what is it?" and we will say "it is an element that only lasts for few seconds, it isn't anything you have seen or anything" and they will say "then why do we have to know about it?" and we will sigh, and try to remember why we thought it would be fun to be a science teacher.....
Want to find out more?
Element 112 is Named Copernicium (official IUPAC News Brief)
Element 112 Gets a "Planetary" Namesake
Sunday, March 7, 2010
Confounding compounds
We know that an element is the simplest form of matter. You can't break it down any smaller with doing some kind of nuclear reaction and those tend to not be very compatible will being alive later o do more chemistry. All of the elements are listed on the Periodic Table, and if it isn't on the Periodic Table it isn't an element (sorry Earth, Wind, Water and Fire). Elements have distinct properties that are different from the properties of all of the other elements. That's how we can tell them apart.
But, something special happens when you get two elements to stick together, they become a new thing with completely different properties than either of the elements you started with. For example, sodium is a metal that reacts explosively with water and chlorine is a very poisonous gas. But, when they are stuck together to make sodium chloride they become a white crystal solid that is actually necessary for life.
These elements-stuck-together are compounds. The elements get stuck together in a couple of different ways, but once they are stuck together you can't get them apart by cutting, or sorting, or filtering or any other physical method. You have to do some kind of chemical reaction to get them back apart. That's what makes them different from just mixtures, where the stuff is mixed together but not chemically stuck together. Mixtures you can take apart without needing a chemical reaction.
Saturday, March 6, 2010
So sodium carbonate plus silver nitrate yields....what now?
It actually isn't as much hocus-pocus as it seems like when you watch the teacher doing the examples on the board. There really are rules you can follow. You do have to know the 5 reactions types first, or you won't be able to get anywhere. Luckily I have already covered that and you can check it out if you need review. Reaction Types, or "What's Going On?" and How Could I Forget Combustion??
So if you start with 2 single elements, it is synthesis and the product will be a simple ionic compound made of those elements. You put the positive one first and use the charges to figure out the subscripts in the formula.
If you start with 1 compound, it is decomposition and the products are the two elements that made up the compound. Remember that diatomic elements get a little subscript 2 when they are alone.
If you start with 1 compound and one element you have a single replacement reaction. If the element is a metal it will switch places with the metal in the compound. If the element is a nonmetal it will switch places with the nonmetal in the compound. You need to check the activity series to see if the reaction will happen. If the element is more reactive (higher on the list) that what it will switch with in the compound the reaction will happen. If it is less reactive the reaction will not happen. (The "No Reaction" ones are easy because you don't have to figure out any formulas or balance anything)
If you start with 2 compounds, it is a double replacement and you will switch the two metals. Each metal will pair up with the other nonmetal or polyatomic ion.
Whenever you make a compound you need to look at the charges to make sure you get the correct formulas.
If you start with a hydrocarbon (something with just hydrogen and carbon) plus oxygen, it is combustion and the products will always be carbon dioxide and water.
So, figure out what type of reaction it is and follow the rules to figure out you will get for products.
Friday, March 5, 2010
How Could I Forget Combustion??
 It has been brought to my attention that in my recent post about reaction types I forgot explain the combustion reaction. This should be surprising to anyone who knows a chemistry teacher because most of us went in to this career so we could blow stuff up whenever we want...
It has been brought to my attention that in my recent post about reaction types I forgot explain the combustion reaction. This should be surprising to anyone who knows a chemistry teacher because most of us went in to this career so we could blow stuff up whenever we want...Or at least that's part of the reason. But seriously, most science teacher are kind of pyromaniacs. So it seems surprising that I could have forgotten the type of reaction at the very heart of burning stuff!
Combustion reactions (fire!) are a chemical reaction between a hydrocarbon and oxygen. "Hydrocarbon" is a fancy word for a compound made only of carbon and hydrogen in some combination. This includes things most of us have heard of, like methane, propane and butane. They always produce carbon dioxide and water, which makes them easiest of all the reaction types to predict products for.

So, there you are. The last (and most fun) reaction type!
Thursday, March 4, 2010
Why do I need to learn this?
This is of course closely followed by "Did we do anything while I was gone?" as the second most annoying question but that is not this post.
So why do we make kids learn all of that stuff anyways? The answer is complicated of course, which is why most students don't actually want to hear it. It is sometimes easier to just tell them that they have to learn it because the politicians say so and they get to decide that stuff.
One of the real reasons is that we teach the increasingly complicated science material in high school is to prepare the students that will be going on to careers in science. If you are going to take a chemistry or physics class in college it is a good idea to take it high school first. And while most high school students are sure they will be professional basketball players or the next American Idol, in reality most of them will get real jobs of some kind. And even some of the students that think they will never take another science class will decide later on that they want to be nurses or engineers.
So in order to prepare the students that will need to take science classes later on, we need to prepare everyone just in case. As you can imagine this answer doesn't really go over well with Mr. future-pro-basketball and Ms. next-American-Idol.
The next reason is that real life as a responsible involved citizen actually requires some basic science knowledge. If someone is trying to sell you something that is supposed to magically remove hard water stains, and you understand the basics of chemical reactions you might be able to decide whether or not to spend $45 dollars for vinegar. And if you know how the water cycle works you will know how to participate in discussions about acid rain or water pollution and how to make informed decisions about voting and policies. We really do need people to understand the science behind all of the tricky political issues that we hear about all of the time.
Somehow students don't actually think this is a good reason, they are pretty sure they can just listen to the television, internet, politician, advertisement, famous person or anyone else and then they don't have to think for themselves.
The last reason, and actually the one I think might be the most important, is that science teaches us how to think. It teaches logical reasoning, problem solving, critical thinking and following directions. Ok, the last one might not actually count as how to think, but I think it is pretty important for people to figure out "Read step 1", "Do step 1", "Read step 2", "Do step 2", etc, etc. This skill seems to be in short supply. And that, along with the other thinking skills are necessary to do a good job no matter what you end up doing. Employers want to hire people who can look at a problem, see multiple ways of solving it, evaluate the possibilities and then solve the problem efficiently. Also, the more you use your brain, the better it works and the longer it works. We know that people who do problem solving and reasoning tasks as they age show less mental deterioration.
This one seems to be the least popular with students, who of course will do whatever they can to avoid thinking...ever.
Wednesday, March 3, 2010
Earthquake made days shorter. No, really....
NASA: Chile Quake Shifted Earth's Axis, Shortened Day
How The Chilean Quake Moved An Entire Planet
Your Day Feel Shorter? Blame Chile's Earthquake
Somehow it kind of shatters a little illusion of constancy that I had. A day is exactly as long a 1 day, that's the whole point. And if the length of a day can change then nothing at all is constant.
Which I really do know. And it isn't as if the change is big enough to make any difference, and it has happened before and it will happen again.
Goodbye 1.26 microseconds of every day...I will miss you! (or not)
Tuesday, March 2, 2010
Reaction Types, or "What's Going On?"
I'm sure you get the idea. There are 5 basic types that most common reactions can be divided into, synthesis, decomposition, single replacement, double replacement and combustion. These sound a lot more confusing than they actually are. We have reactions where things combine, where things come apart, where things change places (2 types) and where things burn. There are also some fancy, complicated organic reactions, but we aren't going to get in to that.
*Reactions where things combine - Synthesis reactions

When two substances (reactants) combine to form a single new compound (product) that is a synthesis reaction. "Synthesis" just means putting things together, and that is all that is happening in these reaction. These are easy to find because they will only have 1 thing after arrow (1 product).
*Reactions where things come apart - Decomposition Reactions
When a single compound (reactant) comes apart and produces 2 or more simpler substances (products) that is a decomposition reaction. Most of us know that decomposition is when stuff comes apart, like whe
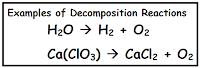 n roadkill on the side of the road slowly gets smaller and smaller over time... You can find these really easily too, just look for reactions with only one thing before the arrow (reactant).
n roadkill on the side of the road slowly gets smaller and smaller over time... You can find these really easily too, just look for reactions with only one thing before the arrow (reactant).*Reactions where things change places - Single and Double Replacement reactions
When you start out with 2 compounds or with 1 compound and 1 element, something is going to change places with something else. These are called replacement reactions because what basically happens is the stronger of the 2 (more reactive) goes in and replaces the weaker (less reactive). It's important to remember that thing only replaces similar things, so metals switch with other metals, nonmetals with nonmetals. We have 2 names for these because 1 describes the 2-compound reactions and one describes the 1-element-and-1-compound reactions.


Monday, March 1, 2010
Earthquake! What's going on?
While it is possible that one of those things is true, it isn't really very likely. And there are actually large earthquakes every year, it's just that these last 2 have caused a lot of damage and injured or killed a lot of people. Large earthquakes that happen in places where few people live happen every year and we barely hear about it because it didn't impact human lives.
On average there is about 1 earthquake of magnitude 8 or greater every year. That is large enough to cause major damage to structures. We have had 2 so far this year, but there were none in 2008 or 2009. In 2007 there were 4 magnitude 8 or greater earthquakes. There are also 12-14 magnitude 7.0-7.9 earthquakes every year. Those can still cause large amounts of damage, particularly in areas with low quality construction (like Haiti).
Every year there are thousands of earthquakes with smaller magnitudes, up to 15,000 large enough to noticed by people near the epicenter. That is a lot of earthquakes, and most of them we never hear anything about.

